The link between our gut and brain is something we’ve all felt. Think about the butterflies in your stomach when you feel excited, or the nausea and loss of appetite that can come with intense anxiety. These are examples of how our emotions directly affect our gut, showing the close relationship between our digestive system and brain.
Our gut isn’t just sensitive to emotions but also to our thoughts and actions. For example, certain types of therapy, like cognitive behavioral therapy, highlight how connected our thoughts, feelings, and actions are, which also plays into gut health.
Not only does our mental state impact our gut, but it also works the other way around: gut health affects our brain. Let’s consider a couple of fascinating facts to get started:
1. Our gut contains around 200 million neurons.
2. Approximately 90-95% of our body’s serotonin (a key neurotransmitter influencing mood) is produced in the gut.
How the Gut and Brain Communicate: Key Players in the Gut-Brain Axis [1]
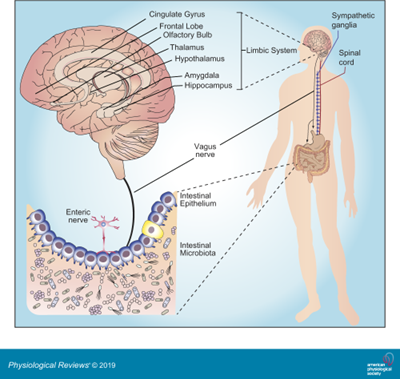
The gut-brain axis is primarily composed of:
- Central Nervous System (CNS)
- Autonomic Nervous System (ANS)
- Enteric Nervous System (ENS)
- Hypothalamic-Pituitary-Adrenal (HPA) Axis
Communication between the brain and gut occurs through the ANS, primarily via the vagus nerve. It regulates heart rate, blood pressure, digestion, and even speech.
This bidirectional communication system functions through the release of hormones into the bloodstream and also involves the immune system, allowing the brain to recieve signals from the digestive system.
Our gut microbiota, the trillions of bacteria and other microorganisms in the gut, also influences physical and mental health, showing how crucial gut health is for overall well-being.
How Does It Work? Understanding the Enteric Nervous System and Gut Functions [1]
The ENS is often called the “second brain” due to its complex network of nerves that controls digestion and other gut functions. It receives information from the gut lining and sends it back to the brain.
The vagus nerve plays a central role in managing gut function, including motility, mucus secretion, and blood flow. Here’s how it works:
- Parasympathetic Stimulation: Increases gastrointestinal motility and secretion.
- Sympathetic Stimulation: Decreases gastrointestinal activity, balancing our digestive function based on current needs.
Key Functions of the Gastrointestinal Tract
The gastrointestinal tract has two primary roles:
1. Digestion and Absorption: Breaking down food and absorbing nutrients.
2. Defense: Acting as a protective barrier, preventing harmful substances, virus, bacteria and pro-inflammatory molecules from entering the bloodstream.
Layers of Defense
From the outermost to innermost layers, the gut’s protective barrier consists of:
- Microbiota
- Mucus
- Epithelial Cells
- Immune Cells
This selective barrier allows beneficial nutrients to pass through while blocking harmful agents.
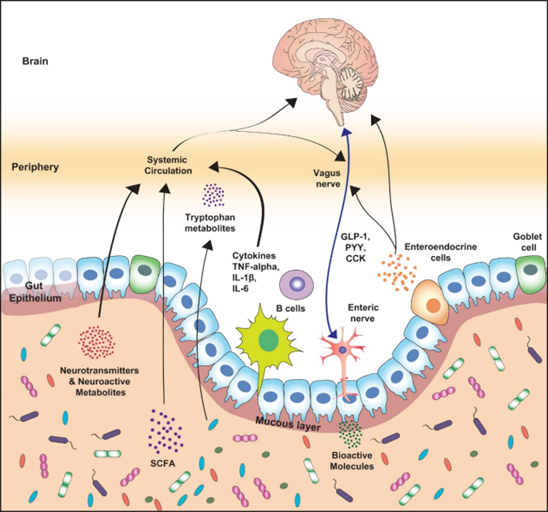
Gut Health and immunity
The gut’s immune system distinguishes between harmless substances and harmful invaders. When a threat is detected, it triggers a defense response, launching inflammatory reactions as needed. The microbiota and mucus are essential parts of this immune barrier, not only protecting against infections but also preventing the passage of toxins into the bloodstream.
However, disruptions in this finely tuned system can lead to conditions like:
- Irritable bowel syndrome (IBS).
- Candida overgrowth.
- Small intestinal bacterial overgrowth (SIBO).
- Celiac disease
These imbalances affect the motility and secretion, can trigger immune responses, increase intestinal permeability, and activate pain pathways.
Gut Inflammation Impacts Brain Health
Every time we eat, a mild inflammatory response is triggered as a precautionary measure, usually resolving after digestion. However, chronic inflammation from frequent eating or consuming certain foods can create an inflammatory environment. When inflammation becomes chronic ,it can send stress signals to the brain, which can lead to symptoms like:
- Fatigue and low energy.
- Fever.
- Depressive symptoms like apathy.
The Microbiota: A Major Player in Our Gut-Brain Connection
Our microbiota consists of approximately 10 trillions of microorganisms, including bacteria, protozoa, fungi, yeasts, and parasites, all densely colonized in the gut. Although once believed to be about 90% bacterial cells, the new evidence suggests a closer 50-50 ratio with human cells. [2]
It means that for every human cell, there’s approximately one bacterial cell.
Microbiota’s Role in Development and Brain Function
Studies show that bacterial colonization in the gut is crucial for the development and maturation of the brain and the ENS. Absence of this colonization is linked to altered neurotransmitter expression, leading to delays in gastric emptying and intestinal transit. [3]
Research also links gut dysbiosis (imbalance in microbial populations) with mental health disorders like:
- Depression.
- Anxiety.
- Neurodegenerative conditions such as Alzheimer’s and Parkinson’s disease
Dysbiosis can influence gastrointestinal motility, secretion, and even visceral sensitivity, which can have significant effects on behavior and emotional health. [4], [5], [6], [7]
Microbiota and Human Behavior
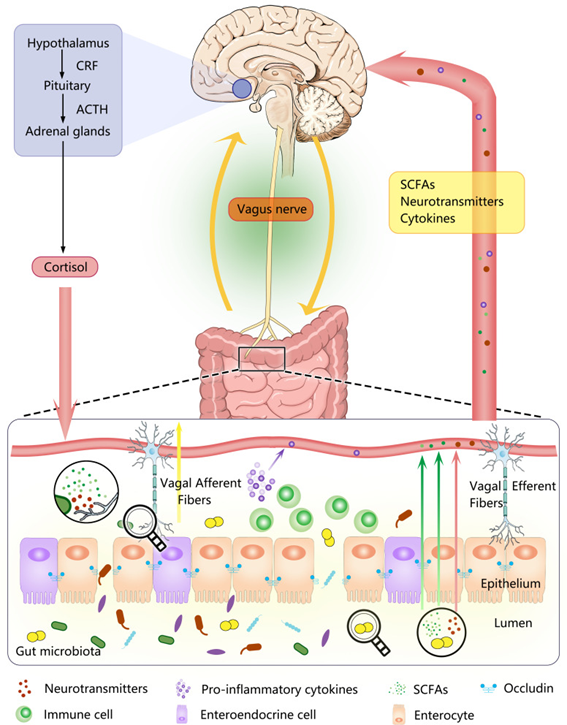
Changes in the microbiota can impact mood and behavior, and conversely, behavioral changes or stress can alter the microbiota’s composition. The microbiota communicates with the brain through:
- The Vagus Nerve: it detects changes in the microbiota and guts and transmits them to the brain. Research has shown that stimulating the vagus nerve may help treat conditions like depression, chronic pain, anxiety, and stress.
Additionally, studies administering Lactobacillus rhamnosus demonstrated increased transcription of GABA, which altered behavior dependent on vagal integrity. [8]
- Neurohormones: serotonin, dopamine, GABA, and catecholamines, largely produced in the gut, also play a role in mood and behavior.
Serotonin is produced in the gut and regulated by the microbiota, it impacts our mood by regulating precursors like tryptophan, whose production is regulated by enzymes metabolized by the intestinal microbiota.
Dopamine production is also linked to the microbiota. Studies using germ-free mice (without any microbiota) have shown reduced dopamine levels, which is an intriguing area of research in understanding the pathogenesis of Parkinson’s disease. [4], [9]
Furthermore, the microbiota aids in GABA production an essential neurotransmitter for behavioral modulation. Studies indicate that probiotic supplementation, which increases GABA availability, can improve anxiety control, underscoring the microbiota’s role in mental health. [10] - Short-Chain Fatty Acids (SCFAs): produced through the microbiota digestion of dietary fibers, SCFAs (such as propionate, butyrate, and acetate), help to protect the brain (supporting the blood brain barrier integrity) and influence neurotransmitters like GABA, glutamate, and glutamine. [11]
- The hypothalamic-pituitary-adrenal (HPA) axis regulates our response to stress.
For example, high levels of Lactobacillus rhamnosus have been associated with lower corticosterone (a stress hormone) levels, better stress management, lower depression, and reduced inflammation. Conversely, small stress exposures can impact the microbiota, showing how deeply interconnected these systems are. [11] - Releasing anti-inflammatory substances (cytokines), which can cross the blood brain barrier and stimulate the growth of new brain cells (neurogenesis). This is thought to be neuroprotective, especially following events like ischemic injury.
When Dysbiosis Strikes
When an imbalance occurs in the microbiota, concentrations of important molecules can be altered. This may contribute to the development of various diseases:
- Behavioral and anxiety disorders.
- Alzheimer’s, Parkinson’s.
- Schizophrenia and autism.
Understanding and maintaining a healthy microbiota is not only crucial for gut health but may also be key to supporting emotional and cognitive well-being. [4], [12], [13], [14]
Emotional Eating and Obesity: Gut Microbiota’s Role
In overweight and obese individuals, studies reveal a dysbiotic gut profile, often with reduced microbial diversity. Obesity-related dysbiosis leads to metabolic complications, affecting immunity, energy regulation, gut hormones, and inflammatory processes. [15]
We know that produced by gut bacteria, SCFAs like butyrate, propionate, and acetate help regulate glucose, appetite, and lipid metabolism. Butyrate, for example, supports colon cell health, while propionate regulates satiety through liver functions, and acetate influences cholesterol metabolism and appetite regulation. [16]
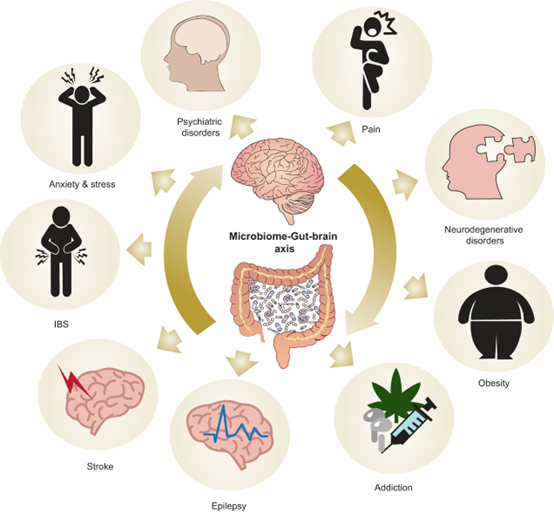
Stress and Anxiety: The Impact on the Gut-Brain Axis
Prolonged exposure to stress can lead to elevated blood cortisol levels (stress hormone). This increase in cortisol can disrupt key brain regions and affect the hypothalamic-pituitary-adrenal (HPA) axis’s ability to regulate stress.This imbalance makes it harder for the brain to manage stress effectively and keeps cortisol levels high, which can lead to gut issues like irritable bowel syndrome (IBS). Research has shown that people with diarrhea-predominant IBS tend to have higher cortisol responses than those with constipation-predominant IBS or healthy people. [17], [18]
Stress also influences gastrointestinal functions, impacting permeability, motility, visceral sensitivity, blood flow, secretions, and microbiota composition. Mechanisms underlying these alterations include increased cortisol, activation of the sympathetic nervous system, dysfunction of the gut barrier, and immune cell stimulation. Stress is associated with several gastrointestinal issues, such a:
Acute stress events, like public speaking, are known to alter gastric acid and mucus secretions, which can increase intestinal permeability and discomfort.
Recent studies reveal that early-life stress, such as maternal separation in neonates, can lead to increased serotonin production in the gut, correlating with IBS symptoms, especially in diarrhea-predominant cases. [19]
About microbiota, it has been shown that in infants, maternal stress during pregnancy has been linked to dysbiosis, characterized by elevated Proteobacteria and decreased beneficial bacteria, leading to:
- Gastrointestinal symptoms.
- Allergies.
- Heightened risk of neurodevelopmental disorders.
Furthermore, galanin, a neuropeptide present in the gut and brain, stimulates cortisol release and affects gut motility, pancreatic function, and growth hormone levels. Furthermore, the gut microbiota can activate the mucosal immune system, a process critical in intestinal immune disorders.
In the context of the brain-gut-microbiota axis, the brain manages gut functions like motility, secretion, and immune responses. Stress disrupts these functions, altering mucus quality and quantity, which impacts microbial habitat in the gut.
The gut microbiota also affects gamma-aminobutyric acid (GABA) production, a neurotransmitter that calms brain activity and alleviates anxiety. Due to its calming effects, GABA is frequently found in supplements intended for managing anxiety and improving sleep quality.
Interestingly, research suggests that women consuming probiotics may exhibit reduced sensitivity to negative emotional stimuli, highlighting the brain-gut connection. Additionally, certain bacterial strains, such as Campylobacter jejuni, have been linked to heightened anxiety, illustrating how microbiota imbalances can impact mental health. [20]
The Role of Melatonin in Gut Health and Stress [21]
Melatonin is best known for helping us sleep, but it also plays an important role in gut health. While it is synthesized and released by the pineal gland into the bloodstream, reaching peak secretion at night, melatonin levels in the gastrointestinal tract are approximately 400 times higher than in the pineal gland.
Research indicates that melatonin helps prevent and heal stress-induced ulcers by acting on the gut directly. For instance, a study showed that luminal melatonin stimulates the release of bicarbonate in the duodenum to neutralize gastric acid entering from the stomach, a key response to stress-induced acidity.
Additionally, melatonin reduces inflammation in the gut by neutralizing reactive oxygen species and inducing antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX). [22], [23]
Melatonin’s protective effects against stress-induced gastrointestinal injury are remarkable. It acts as an antioxidant, reduces inflammation, and modulates gut motility. In humans, an 8-week course of oral melatonin has been shown to alleviate abdominal pain, bloating, and defecation issues in individuals with irritable bowel syndrome (IBS). [24], [25]
Irritable Bowel Syndrome (IBS) and Its Multifactorial Etiology
IBS is a prevalent functional gastrointestinal disorder, affecting 10-20% of the global population. It is often associated with stress and is linked to environmental factors, psychosocial stress, gut inflammation, and microbiota imbalances. IBS patients typically exhibit heightened sympathetic activity and reduced parasympathetic function, which affects gut motility and secretion. In severe stress, vagal overactivation can even lead to gastrointestinal bleeding and peptic ulcers.
In diarrhea-predominant IBS patients, certain probiotic strains have been shown to reduce intestinal permeability, highlighting the therapeutic potential of microbiota modulation. [17], [18]
Gut Health and other mental Disorders: Alzheimer’s, Depression, and Autism
In Alzheimer’s patients, probiotic supplementation has shown potential in improving cognitive function. [26], [27]
Dopamine is crucial for regulating motivation, pleasure, relaxation, and memory duration. Research involving germ-free mice (mice without microbiota) has shown that these animals have lower levels of dopamine, indicating the gut microbiota’s influence on its production.
Additionally, studies comparing the gut microbiota of 37 individuals with depression to that of 18 healthy controls revealed distinct microbial patterns. Depressed individuals had a lower abundance of Bacteroidetes and a higher presence of the genus Alistipes, suggesting a potential link between gut microbial composition and mood disorders. [28], [29]
In autism, studies suggest a distinct microbiota profile in affected children, with notable differences compared to neurotypical children. This line of research holds potential for new therapeutic approaches to manage autism and related symptoms. [30], [31]
Conclusions
The concept of isolated systems within the human body is increasingly outdated. We’ve explored how interconnected the body’s systems truly are, and how a holistic perspective enhances our understanding of health and well-being. Viewing the body as an integrated whole, rather than merely a collection of separate parts, allows for deeper insights into its complex functions and interdependencies.
There are also other fascinating “axis” within the body that significantly impact mental health, and exploring those could shed even more light on holistic wellness approaches.
If you found this article insightful and are curious about specific ways to diagnose, treat and improve this axis let me know, I’d be glad to dive deeper into practical strategies in a future article.
Discover the Benefits of Finding Your Therapist with It’s Complicated
- Affordable: Our mission is to make therapy accessible to everyone by offering flexible pricing options, so you can find professional support that fits your budget.
- Diverse: Explore our directory of over 1,500 therapists, psychologists, and coaches. Use smart filters for location, language, specialisation, and more to find a therapist that’s right for you.
- Personalised: Need extra help finding the right therapist? Our free matching service connects you with your perfect match, tailored to your specific needs.
- Flexible: Counselling on It’s Complicated is available both online and in-person in over 60 languages, with no waiting times.
It’s Complicated is a therapy platform that connects clients with the right therapist and empowers over 1,500 mental health professionals worldwide. Because life is complicated, but finding a therapist shouldn’t be. If you find yourself in immediate danger or require urgent help, please contact emergency services or use one of these resources instead.
References
1. The Microbiota-Gut-Brain Axis (2018) https://journals.physiology.org/doi/full/10.1152/physrev.00018.2018?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org](https://journals.physiology.org/doi/full/10.1152/physrev.00018.2018?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
2. Revised Estimates for the Number of Human and Bacteria Cells in the Body (2016)
https://pmc.ncbi.nlm.nih.gov/articles/PMC4991899
3. Microbiota and neurodevelopmental windows: implications for brain disorders (2014)
https://pubmed.ncbi.nlm.nih.gov/24956966
4. Gut and Brain: Investigating Physiological and Pathological Interactions Between Microbiota and Brain to Gain New Therapeutic Avenues for Brain Diseases (2021)
https://pmc.ncbi.nlm.nih.gov/articles/PMC8545893
5. Role of the gut microbiota in nutrition and health (2018)
https://pmc.ncbi.nlm.nih.gov/articles/PMC6000740
6. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems (2015) https://pubmed.ncbi.nlm.nih.gov/25830558/
7. The Human Intestinal Microbiome in Health and Disease (2016) https://www.nejm.org/doi/full/10.1056/NEJMra1600266
8. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases (2022)
https://pmc.ncbi.nlm.nih.gov/articles/PMC9656367
9. Changes of Dopamine and Tyrosine Hydroxylase Levels in the Brain of Germ-free Mice (2023)
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/36811101
10. Probiotic, Prebiotic, and Brain Development (2018) https://pubmed.ncbi.nlm.nih.gov/29135961/](https://pubmed.ncbi.nlm.nih.gov/29135961/
11. Specialized metabolites from the microbiome in health and disease (SCFA) (2014)
https://pubmed.ncbi.nlm.nih.gov/25440054
12. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health (2019)
https://pmc.ncbi.nlm.nih.gov/articles/PMC6469458
13. Intestinal microbiota and mental behavior disorders (2020)
http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0034-75312020000200016
14. Cognitive-Behavioural Correlates of Dysbiosis: A Review (2020)
https://pmc.ncbi.nlm.nih.gov/articles/PMC7402132
15. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics (2022)
https://pmc.ncbi.nlm.nih.gov/articles/PMC8877435/
16. The role of short chain fatty acids in appetite regulation and energy homeostasis (2015)
https://pmc.ncbi.nlm.nih.gov/articles/PMC4564526
17. Pathophysiology underlying irritable bowel syndrome -From the viewpoint of dysfunction of autonomic nervous system activity (2014) https://www.jstage.jst.go.jp/article/jsmr/45/1/45_1_15/_pdf/-char/en
18. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? (2014)
https://pmc.ncbi.nlm.nih.gov/articles/PMC4202342
19. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner (2012)
https://www.nature.com/articles/mp201277
20. Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity (2013)
https://www.gastrojournal.org/article/s0016-5085(13)00292-8/fulltext
21. Distribution, function and physiological role of melatonin in the lower gut (2011)
https://pmc.ncbi.nlm.nih.gov/articles/PMC3198018
22. Melatonin in the duodenal lumen is a potent stimulant of mucosal bicarbonate secretion (2002)
23. Antioxidant Actions of Melatonin: A Systematic Review of Animal Studies (2023)
https://pmc.ncbi.nlm.nih.gov/articles/PMC11047453
24. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double-blind, placebo-controlled study (2005) https://pmc.ncbi.nlm.nih.gov/articles/PMC1774717/
25. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind, placebo-controlled study (2005) https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2036.2005.02673.x
26. Probiotics for Alzheimer’s Disease: A Systematic Review(2022) https://pmc.ncbi.nlm.nih.gov/articles/PMC8746506/
27. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial (2016)
https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2016.00256/full
28. Altered Composition of Gut Microbiota in Depression: A Systematic Review (2020)
https://pmc.ncbi.nlm.nih.gov/articles/PMC7299157/#B25
29. Correlation between the human fecal microbiota and depression (2015)
https://pubmed.ncbi.nlm.nih.gov/24888394
30. Multi-level analysis of the gut–brain axis shows autism spectrum disorder-associated molecular and microbial profiles (2023) https://pmc.ncbi.nlm.nih.gov/articles/PMC10322709/
31. Global prevalence of autism spectrum disorder and its gastrointestinal symptoms: A systematic review and meta-analysis (2022) https://pmc.ncbi.nlm.nih.gov/articles/PMC9445193/
